When Diluting a Solution Which of the Following Changes
Diluting a sample will reduce the molarity. The enthalpies of hydration are always negative.
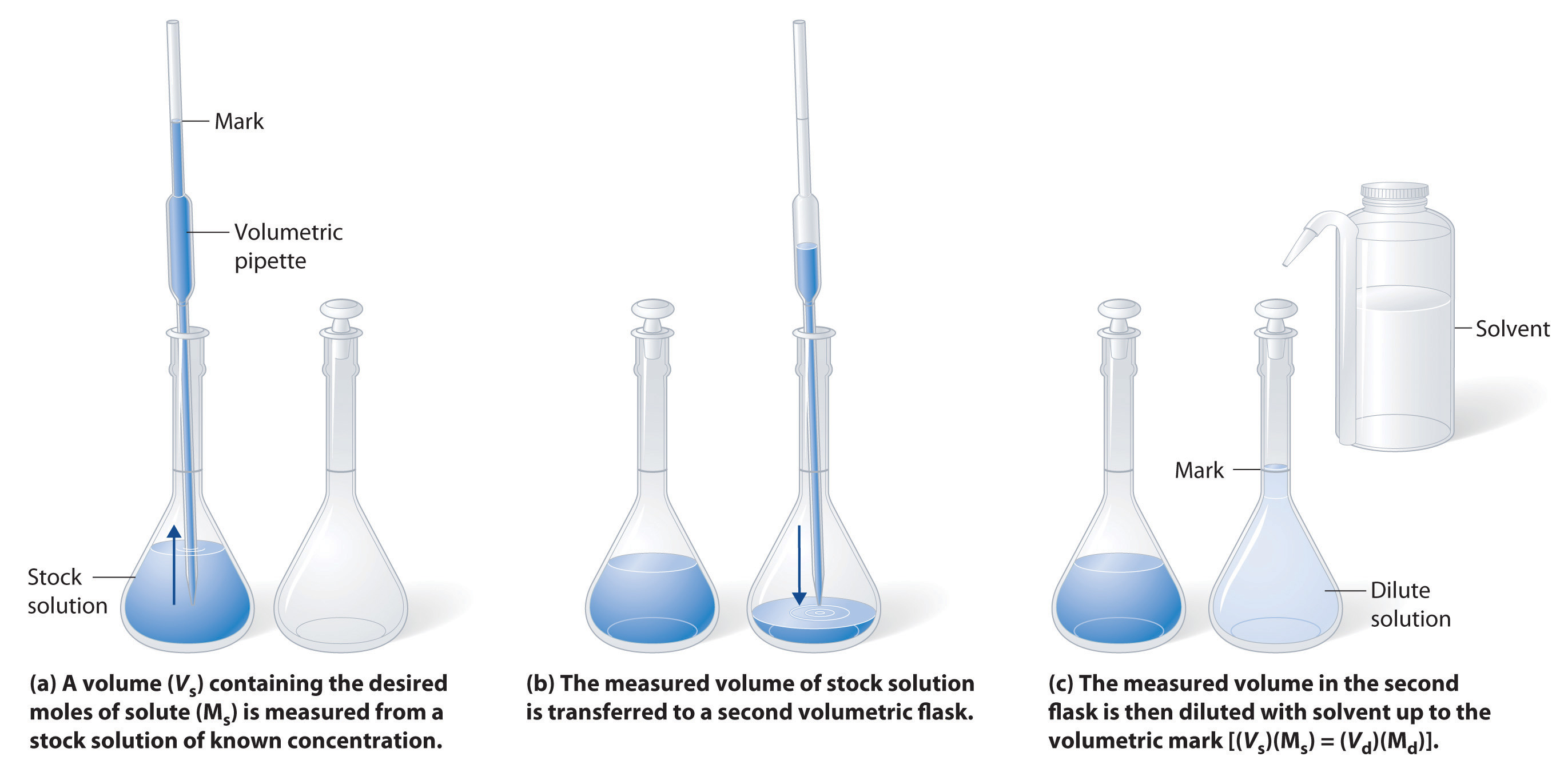
Ch104 Chapter 7 Solutions Chemistry
This new solution will have your desired concentration C 2.
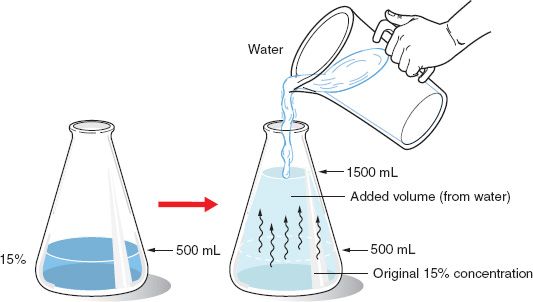
. C 2 Final concentration of diluted solution. The final volume of the solution increases. The enthalpy change that occurs when a solution containing 1 g mole of a solute is diluted from one concentration to another is known as integral enthalpy of dilution.
The acid is becoming less acidic. 1 M 01 M 001 M 0001 M and so on. Contrastly concentrating a solution means to add more of the solute into the solution.
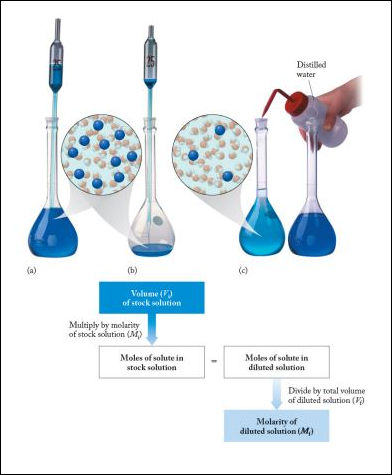
Typically the dilution factor remains constant for each dilution resulting in an exponential decrease in concentration. When you know all four values in the equation C 1 V 1 C 2 V 2 perform your dilution as follows. When you dilute a solution what happens to the molarity.
For example if you have 5mL of a 2M solution which is diluted to a new volume of 10mL the molarity will be reduced to 1M. For example a ten-fold serial dilution could result in the following concentrations. Which of the following stays constant when diluting a solution.
C 1 Concentration of starting solution. See answer 1 Best Answer. The amount of solvent changes.
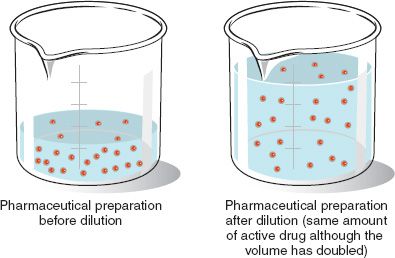
The amount of solute changes. C1 x V1 C2 x V2. The number of moles of solute will not change.
What is diluting solutions. Serial dilutions involve diluting a stock or standard solution multiple times in a row. Volume molarity O concentration amount of solute.
Select the correct answer below. Then add enough diluting liquid water etc to make a total volume V 2. Alkanes and aromatic compounds do not react with potassium permanganate.
04 x V1 25x10-3 x 50. V 2 Final volume of diluted solution. The total number of moles of solute remains unchanged upon dilution so you can write this equation.
When 1 mole of gaseous ions dissolved in enough water to make an infinitely dilute solution the hydration enthalpy changes. Calculate the volume in liters and to the hundredths place of a stock solution that has a concentration of 0235 M CaNO32 and when diluted to a 0872 L becomes 018 M CaNO32. Too the molarity of the solution decreases.
A stock solution has a concentration of 15 M SO2 and is diluted to a 054 M solution with a volume of 018 L. Prepare 50 ml of a 25x10-3M from prepared 04M HCl. When an acidic solution is diluted with water the concentration of H ions decreases and the pH of the solution increases towards 7.
When a purple solution of the oxidizing agent KMnO4 is added to an alkene the alkene is oxidized to a diol and the KMnO4 is converted to brown MnO2. When a concentrated stock solution is diluted Does the mole change. Diluting a solution simply means adding more solvent into the solution.
1 Volume of starting solution needed to make the diluted solution. The final concentration of the solution will be lesser than the initial concentration of the. To make the pH change by 1 a tenfold dilution is required eg adding 9 cm 3 of water to 1 cm 3 acid.
Measure the volume V 1 of the solution with concentration C 1. Thus if the purple color changes to brown in this reaction it is a positive reaction. How does diluting a solution affect pH.
Which of the following is TRUE about dilution. Making Dilutions Diluting a solution reduces the number of moles of solute per unit volume but the total number of moles of solute in solution does not change.

Dilution And Concentration Basicmedical Key
No comments for "When Diluting a Solution Which of the Following Changes"
Post a Comment